Ukongeza kwitekhnoloji, i-synthesis ye-glycosides ibisoloko inomdla kwisayensi, njengoko kuyindlela eqhelekileyo yokusabela kwindalo. Amaphepha akutshanje kaSchmidt noToshima noTatsuta, kunye neembekiselo ezininzi ezikhankanyiweyo apho, baye bagqabaza kuluhlu olubanzi lwezinto ezinokubakho zokwenziwa.
Kwi-synthesis ye-glycosides, amacandelo amaninzi eswekile adityaniswa kunye ne-nucleophiles, njenge-alcohols, i-carbohydrates, okanye iiprotheni, ukuba impendulo ekhethiweyo kunye neqela le-hydroxyl ye-carbohydrate efunekayo, yonke eminye imisebenzi kufuneka ikhuselwe kwisinyathelo sokuqala. Ngokomgaqo, iinkqubo ze-enzymatic okanye i-microbial, ngenxa yokukhethwa kwazo, zinokuthi zithathe indawo yokukhusela iikhemikhali eziyinkimbinkimbi kunye namanyathelo okukhusela ngokukhetha kwi-glycosides kwimimandla. Nangona kunjalo, ngenxa yembali ende ye-alkyl glycosides, ukusetyenziswa kwee-enzymes kwi-synthesis ye-glycosides akuzange kufundwe ngokubanzi kwaye kusetyenziswe.
Ngenxa yomthamo weenkqubo ze-enzyme ezifanelekileyo kunye neendleko eziphezulu zokuvelisa, i-enzymatic synthesis ye-alkyl polyglycosides ayikulungele ukuphuculwa kwinqanaba lezoshishino, kwaye iindlela zemichiza zikhethwa.
Ngo-1870, i-MAcolley yabika ukuhlanganiswa kwe "acetochlorhydrose" (1,figure2) ngokusabela kwe-dextrose(glucose) nge-acetyl chloride, eyathi ekugqibeleni yakhokelela kwimbali yeendlela ze-glycoside synthesis.
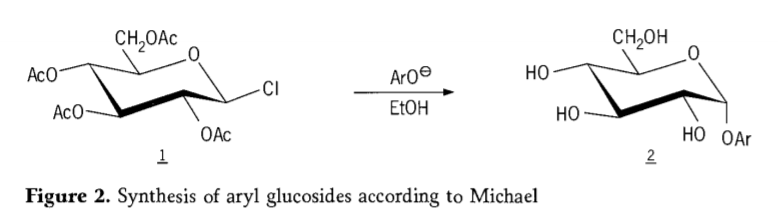
I-Tetra-0-acetyl-glucopyranosyl halides(i-acetohaloglucoses) yafunyanwa kamva njengeziphakathi eziluncedo kwi-stereoelective synthesis ye-alkyl glucosides ecocekileyo. Ngomnyaka we-1879, u-Arthur Michael waphumelela ekulungiseleleni i-aryl glycosides eqinisekileyo, ecacileyo evela kwi-Colley's intermediates kunye ne-phenolates. (Aro-,Figure 2).
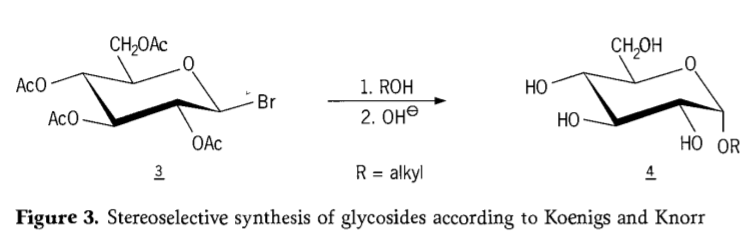
Ngo-1901, ukuhlanganiswa kukaMichael kuluhlu olubanzi lweecarbohydrates kunye ne-hydroxylic aglycons, xa u-W.Koenigs kunye no-E.Knorr bazisa inkqubo yabo ephuculweyo ye-stereoelective glycosidation (Figure 3). I-reaction ibandakanya ukutshintshwa kwe-SN2 kwi-carbon ye-anomeric kwaye iqhube i-stereoselectively kunye ne-inversion ye-configuration, ivelise umzekelo i-α-glucoside 4 ukusuka kwi-β-anomer ye-aceobromoglucose ephakathi 3. I-Koenigs-Knorr synthesis iyenzeka phambi kwesilivere okanye i-mercury promotors.
Ngo-1893, u-Emil Fischer wacebisa indlela eyahlukileyo ngokusisiseko ekudityanisweni kwe-alkyl glucosides. Le nkqubo ngoku yaziwa ngokuba yi "Fischer glycosidation" kwaye ibandakanya ukusabela kwe-acid-catalyzed of glycoses with alcohols. Nayiphi na i-akhawunti yembali kufuneka nangona kunjalo ibandakanye inzame yokuqala ye-A.Gautier exelwe kwi-1874, ukuguqula i-dextrose nge-ethanol e-anhydrous phambi kwe-hydrochloric acid. Ngenxa yohlalutyo olulahlekisayo, uGautier wayekholelwa ukuba ufumene "i-diglucose". UFischer kamva wabonisa ukuba “i-diglucose” kaGautier enyanisweni ubukhulu becala yayiyi-ethyl glucoside (Umfanekiso 4).
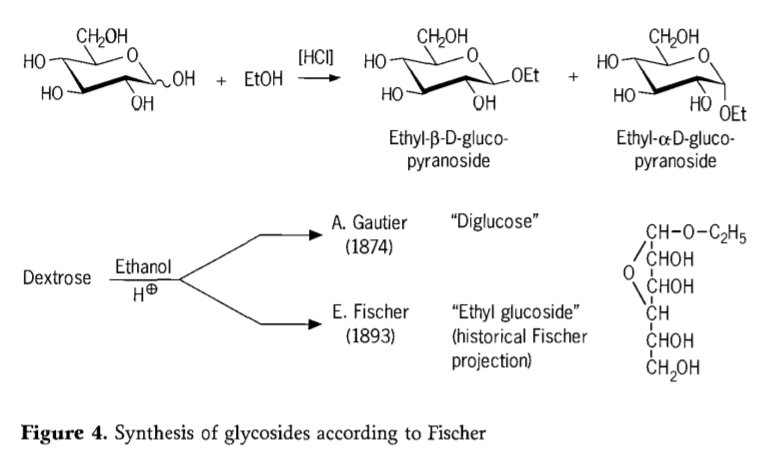
UFischer uchaze ubume be-ethyl glucoside ngokuchanekileyo, njengoko kunokubonwa kwifomula ye-furanosidic yembali ecetywayo. Enyanisweni, iimveliso ze-Fischer glycosidation ziyinkimbinkimbi, ubukhulu becala imixube elinganayo ye-α/β-anomers kunye ne-pyranoside / i-furanoside isomers ekwabandakanya i-oligomers ye-glycoside edibeneyo.
Ngokuhambelanayo, iintlobo zeemolekyuli zomntu akulula ukuzihlukanisa kwimixube yokusabela kweFischer, ebiyingxaki enkulu ngaphambili. Emva kophuculo oluthile lwale ndlela yokudibanisa, uFischer emva koko wamkela i-Koenigs-Knorr synthesis kuphando lwakhe. Ukusebenzisa le nkqubo, u-E.Fischer kunye no-B.Helferich babe ngabokuqala t baxela ukuhlanganiswa kwe-alkyl glucoside ye-chain-chain ebonisa iipropati ze-surfactant kwi-1911.
Kwango-1893, uFischer waye waqaphela ngokuchanekileyo iipropathi ezibalulekileyo zealkyl glycosides, ezinjengozinzo lwazo oluphezulu ngokubhekiselele kwi-oxidation kunye ne-hydrolysis, ngakumbi kumajelo e-alkaline. Zombini iimpawu zibalulekile kwi-alkyl polyglycosides kwizicelo ze-surfactant.
Uphando olunxulumene ne-glycosidation reaction lusaqhubeka kwaye iindlela ezininzi ezinomdla kwi-glycosides ziye zaphuhliswa kutsha nje. Ezinye zeenkqubo zokwenziwa kwe-glycosides zishwankathelwa kuMfanekiso 5.
Ngokuqhelekileyo, iinkqubo ze-chemical glycosidation zinokwahlulwa zibe ziinkqubo ezikhokelela kwi-oligomer equilibria enzima kwi-acid-catalysed glycosyl exchange.
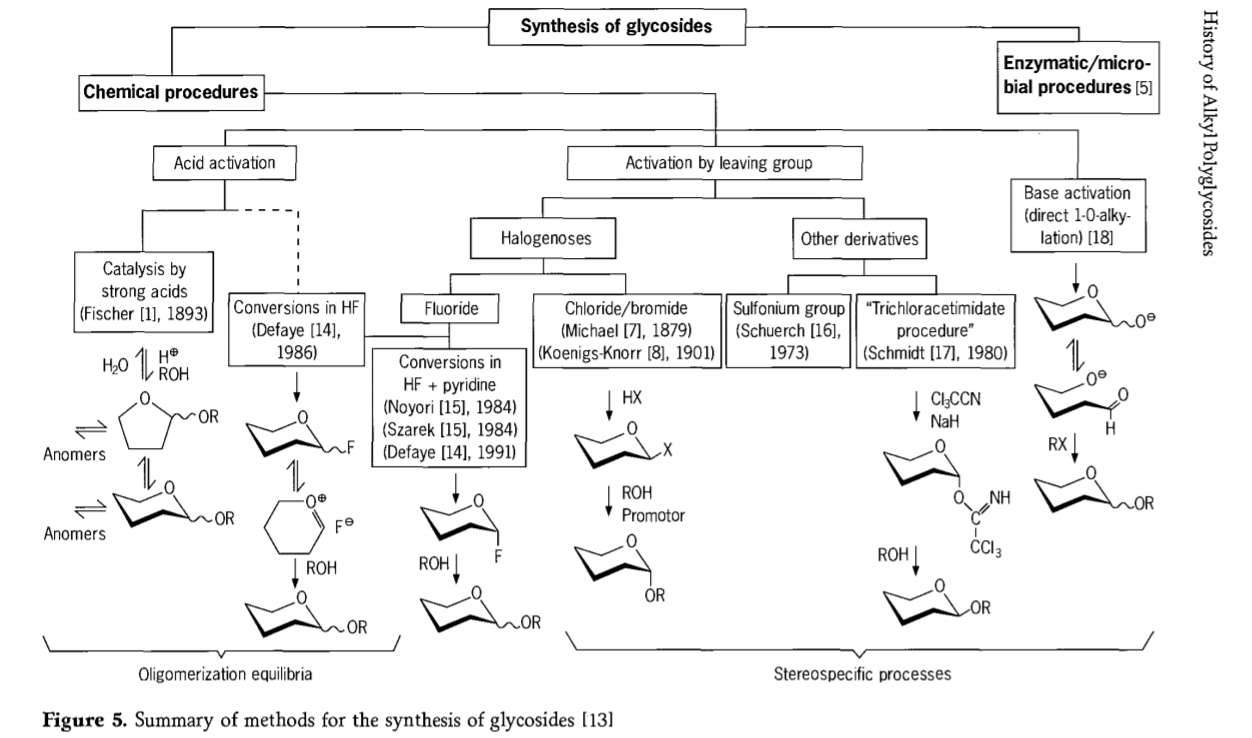
Iimpendulo kwii-substrates ze-carbohydrate ezenziwe ngokufanelekileyo (i-Fischer glycosidic reactions kunye ne-hydrogen fluoride (HF) reactions kunye ne-molecule ze-carbohydrates ezingakhuselekanga) kunye ne-kinetics elawulwayo, engenakurhoxiswa, kwaye ubukhulu becala i-stereotaxic substitution reactions. Uhlobo lwesibini lwenkqubo lunokukhokelela ekwenziweni kohlobo oluthile kunokuba lube kwimixube entsonkothileyo yokusabela, ngakumbi xa zidityaniswe nobuchule beqela lolondolozo. Iicarbohydrate zinokushiya amaqela kwi-ectopic carbon, njenge-athomu ye-halogen, i-sulfonyls, okanye amaqela e-trichloroacetimidate, okanye isebenze ngeziseko ngaphambi kokuguqulwa kwi-triflate esters.
Kwimeko ethile ye-glycosidations kwi-hydrogen fluoride okanye kwimixube ye-hydrogen fluoride kunye ne-pyridine (i-pyridinium poly [hydrogen fluoride]), i-glycosyl fluorides yenziwe kwindawo kwaye iguqulwa kakuhle ibe yi-glycosides, umzekelo ngee-alcohols. IHydrogen fluoride ibonakaliswe njengesixhobo sokusabela esinamandla, esingaphucukiyo; i-equilibrium auto condensation (oligomerization) ibonwa ngokufana nenkqubo ye-Fischer, nangona indlela yokusabela mhlawumbi yahlukile.
I-alkyl glycosides ecocekileyo ngokwekhemikhali ifanelekile kuphela kwizicelo ezikhethekileyo. Ngokomzekelo, i-alkyl glycosides isetyenziswe ngempumelelo kwi-biochemical research ye-crystallization ye-membrane proteins, njenge-crystallization ye-dimensional ye-porin kunye ne-bacteriorhodopsin phambi kwe-octyl β-D-glucopyranoside (ezinye iimvavanyo ezisekelwe kulo msebenzi zikhokelela kwi-Nobel prize kwi-chemistry ye-Deisenhofer 8 e-Huber98, i-Huber98, i-Huber98, i-Huber98).
Ngexesha lophuhliso lwe-alkyl polyglycosides, iindlela ze-stereoselective zisetyenzisiwe kwisikali selabhoratri ukudibanisa izinto ezahlukeneyo zemodeli kunye nokufunda iipropathi zazo ze-physicochemical, ngenxa yokuntsokotha kwazo, ukungazinzi kwe-intermediates kunye nenani kunye nobume obubalulekileyo bokuchithwa kwenkqubo, ukuhlanganiswa kohlobo lwe-Koenigs-Knorr kunye nezinye iingxaki ezibalulekileyo zoqoqosho ziya kudala amaqela abalulekileyo oqoqosho kunye nobuchule. Iinkqubo zohlobo lwe-Fischer ngokuthelekisayo zincinci kwaye zilula ukwenza kwinqanaba lezorhwebo kwaye ngokufanelekileyo, yindlela ekhethiweyo yokuvelisa i-alkyl polyglycosides kwizinga elikhulu.
Ixesha lokuposa: Sep-12-2020





